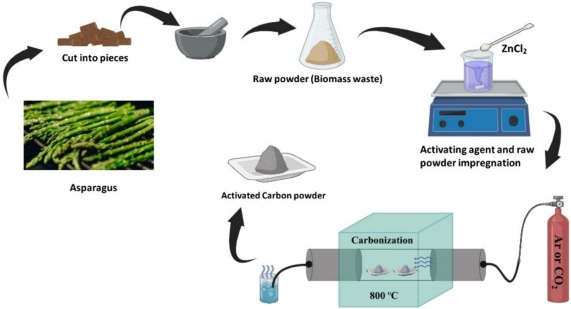
Supercapacitors with carbon electrodes derived from biomass and utilizing gel polymer electrolytes are currently a focal point in the development of highly efficient, environmentally friendly, and cost-effective energy storage devices. In this study, we present porous activated carbon derived from asparagus waste, prepared through chemical activation with ZnCl2 followed by physical activation with CO2, as a high-performance electrode material for supercapacitors. The performance of electrodes has been discussed in comparison with supercapacitors employing both gel polymer electrolytes and conventional liquid electrolytes i.e. 7 M KOH. The flexible film of the gel polymer electrolyte exhibits noteworthy characteristics, including a high ionic conductivity of ∼6.3 mS cm−1, and a high electrochemical stability window of ∼4.5 V. Supercapacitors prepared with this gel polymer electrolyte outperform supercapacitors with liquid electrolytes thanks to a broader electrochemical stability window, showing optimal charge-discharge performance, a specific capacitance of 160 F g−1, a specific energy of 31 Wh kg−1, and an effective power of 0.56 kW kg−1. The superior rate performance is demonstrated by powering a LED for a substantial duration, highlighting the exceptional capabilities of the system. Additionally, the supercapacitor employing the gel polymer electrolyte displays an extended stability, sustaining approximately 10,000 charge-discharge cycles with only a modest initial fading of ∼16 % in specific capacitance and maintaining a high coulombic efficiency of ∼100 %.
Reproduced with permission. Copyright 2024, Elsevier