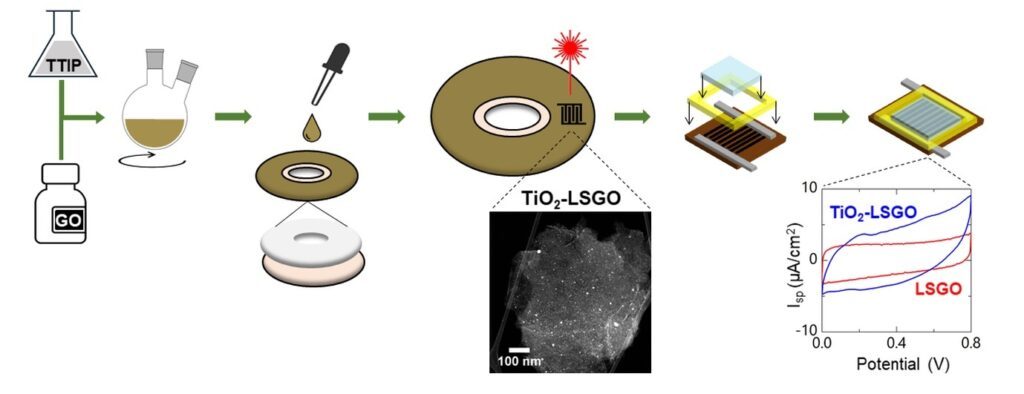
Graphene-based miniaturized supercapacitors, obtained via laser conversion of suitable precursors, have been attracting recent attention for the production of energy storage small-scale devices. In this work, a one-pot synthesis of TiO2 nanoparticles embedded in porous graphene-based electrodes has been obtained with the LightScribe® technology, by converting the precursor materials through the absorption of a DVD burner infrared laser light. Enhanced electrochemical performance of devices has been achieved thanks to the combination of faradic surface reactions, arising from metal oxide nanoparticles, with the conventional electrochemical double layer capacitance, arising from porous graphene. Micro-supercapacitors, consisting of TiO2-graphene electrodes, have been tested by investigating two hydrogel polymer electrolytes, based on polyvinyl alcohol/H3PO4 and polyvinyl alcohol/H2SO4, respectively. Specific areal capacitance up to 9.9 mF/cm2 are obtained in TiO2-graphene devices, corresponding to a volumetric capacitance of 13 F/cm3 and doubling the pristine graphene-based device results. The micro-supercapacitors achieved specific areal energy and specific areal power of 0.22 μWh/cm2 and 39 μW/cm2, along with a cyclability greater than 3000 cycles. These high-performance results suggest laser-scribed TiO2-graphene nanostructures as remarkable candidates in micro-supercapacitors for environment-friendly, large-scale and low-cost applications.
Reproduced with permission. Copyright 2021, Elsevier