Fullerenes
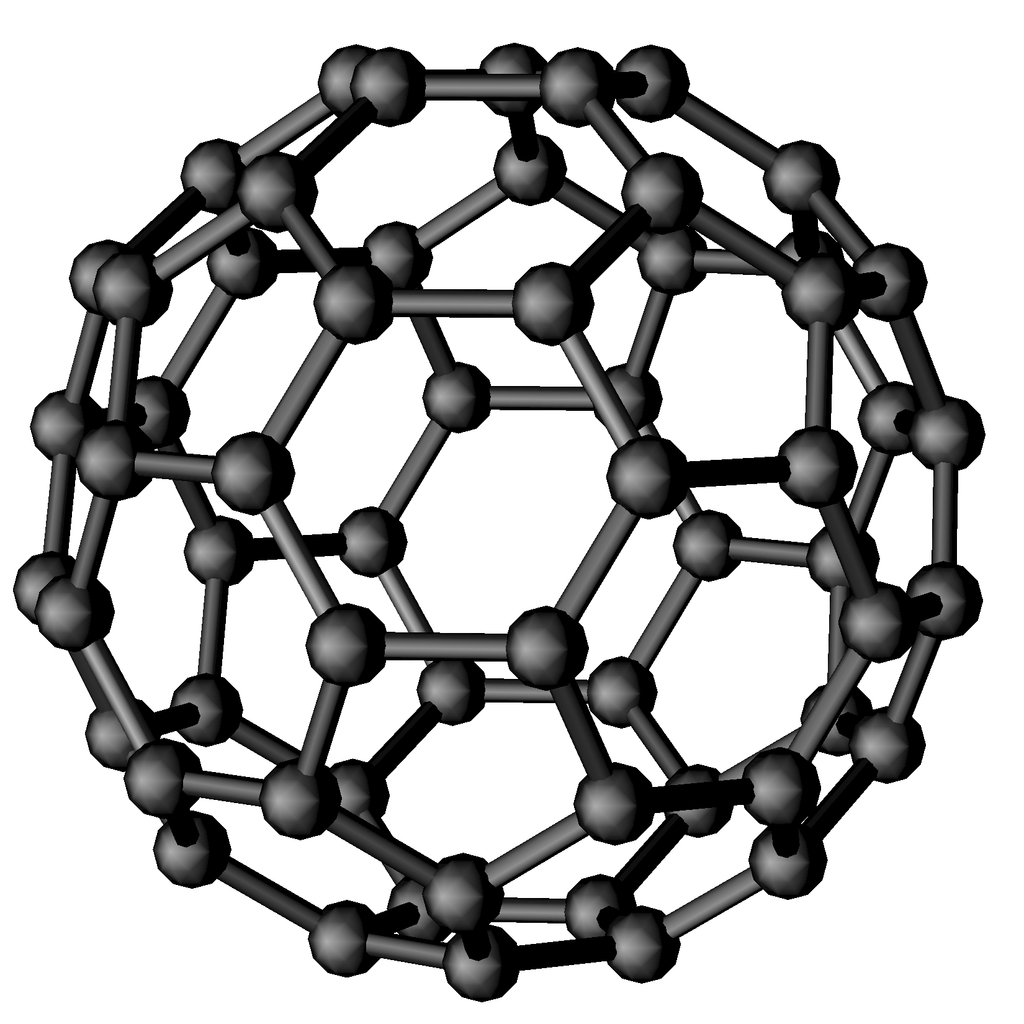
Fullerenes have interesting and peculiar physical and chemical properties and for their discovery R. F. Curl, H. W. Kroto and R. E. Smalley received the Nobel prize for chemistry in 1996. Even if the family includes a large number of fullerene molecules, the most representative and studied one is C60. This molecule is highly symmetrical and has the same shape of a soccer ball, that is, mathematically speaking, a truncated icosahedron. In particular, its surface is made up of 12 pentagons and 20 hexagons. Fullerenes have proved to be important not only for fundamental science, but also for many practical applications, such as the fields of superconductors, lubricants, industrial catalysts, even in drug delivery systems.
In the Nanocarbon laboratory we focus on synthesis of fullerides, systems in which the C60 solid is doped with atoms or molecules, by inserting alkali or alkali-earth metals through solid-state reaction, chemical vapor deposition or reaction in solution. Such compounds have interesting physical properties such as superconductivity with relatively high critical temperatures (up to 38 K in Cs3C60) and ability to ad/absorb hydrogen.
Graphene
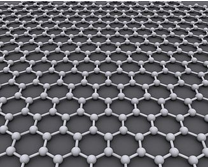
Graphene is a single atomic layer of carbon in the sp2 hybrid state, where atoms are arranged according to the hexagonal order of graphite. In 2004 the group of physicists from Manchester University (UK) led by A. Geim and K. Novoselov obtained for the first time such a mono-atomic layer simply tearing adhesive tape from a crystal of pyrolytic graphite. Their discovery was awarded with the Nobel Prize for Physics in 2011. The unique properties of graphene make it suitable for many types of applications, such as production of field effect transistors, transparent conductors, polymer composites, sensors, high efficiency gas absorber and, last but not least, due to its exceptional specific area, as a support for the storage of hydrogen for future environmentally friendly vehicles.
In the Nanocarbon laboratory graphene is synthesized starting from graphite, which is oxidized in order to increase the distance between layers; graphite oxide is then subject to thermal exfoliation, a violent thermal shock thanks to which layers are separated from each other. This method has the advantage of allowing a very high yield. Graphene is then used as-prepared or functionalized with different kinds of nanoparticles (transition metals, alkali metals, metal oxides), which can be useful to realize catalysts, hydrogen storage systems and electrodes for batteries and supercapacitors.
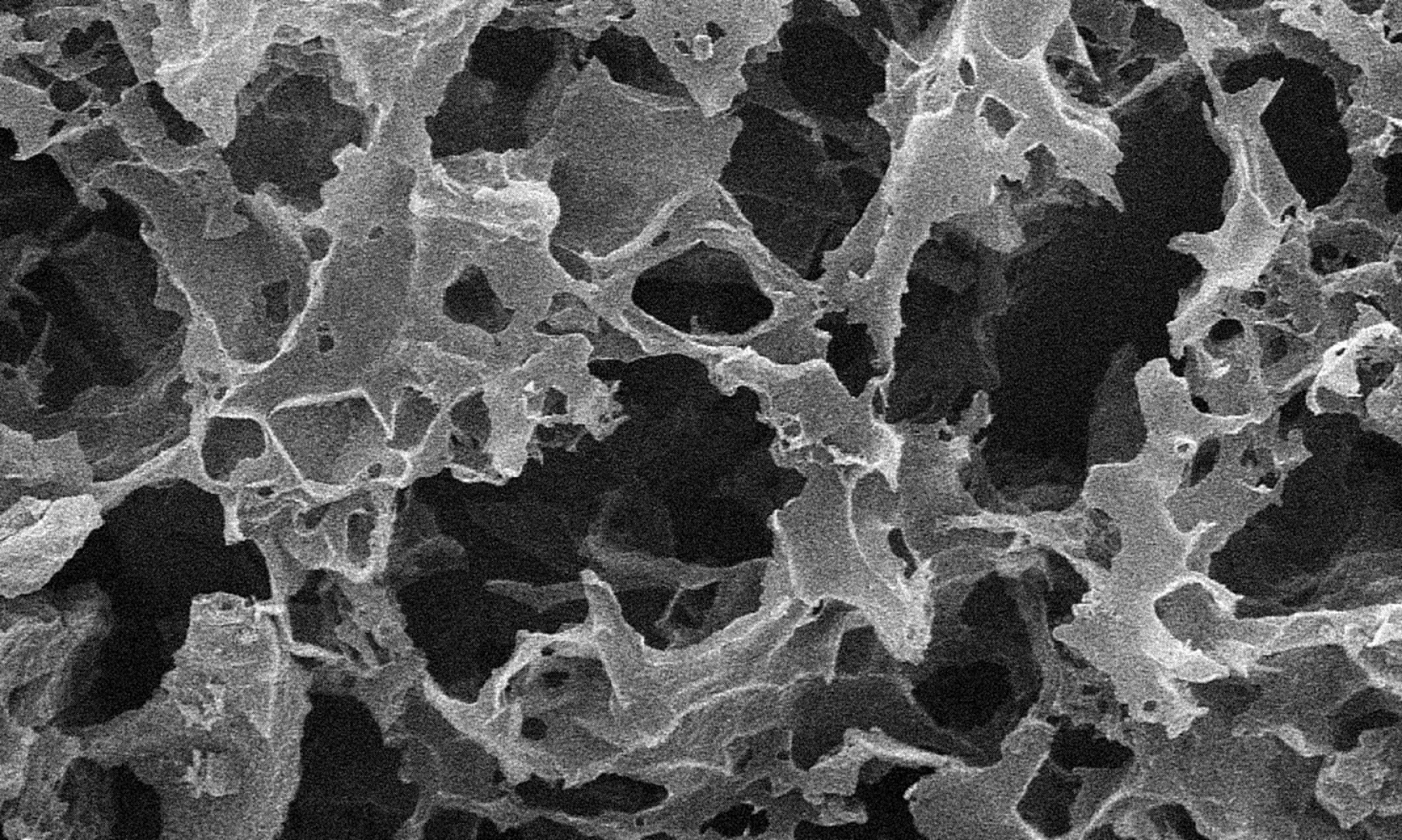
Nanoporous Carbon

Biochar is a charcoal made from biomass via pyrolysis or pyrogassification.
It is made through direct thermal decomposition of biomass in the absence of oxygen, which produces a high-carbon, fine-grained residue. In the Carbon Nanostructure Lab, bio-char is chemically activated in order to increase its specific surface area, up to 3000 m2/g.
Such characteristic makes biochar a good choice as an electrode material for supercapacitors.