Fullerenes
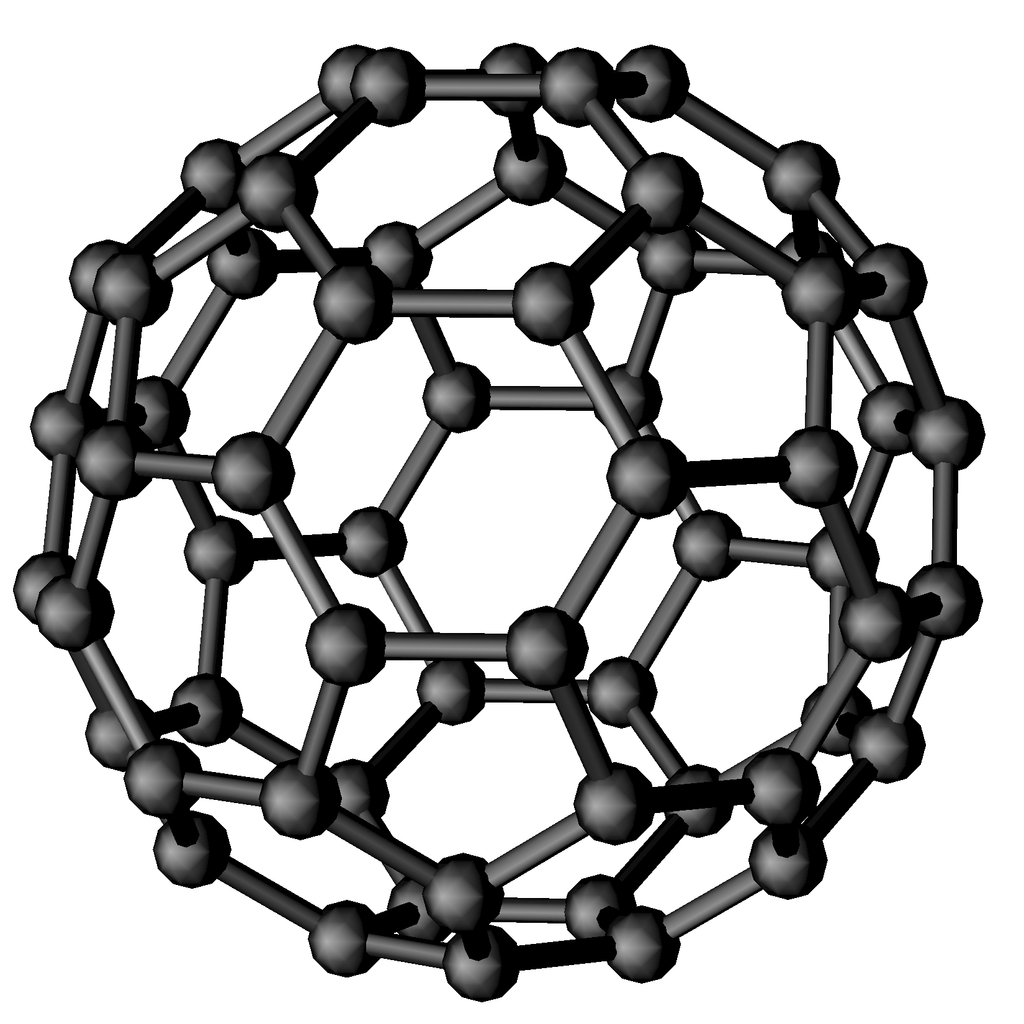
Fullerenes are a unique class of carbon nanostructures with remarkable physical and chemical properties. Their discovery earned R. F. Curl, H. W. Kroto, and R. E. Smalley the Nobel Prize in Chemistry in 1996. Among the many molecules in the fullerene family, the most iconic and widely studied is C₆₀, a highly symmetrical molecule shaped like a soccer ball, or more precisely, a truncated icosahedron, composed of 12 pentagons and 20 hexagons. Fullerenes have attracted considerable interest not only in fundamental research but also in a range of technological applications, including superconductors, lubricants, industrial catalysts, and even drug delivery systems, thanks to their ability to encapsulate or interact with other molecules. At the Nanocarbon Laboratory, research on fullerenes focuses primarily on the synthesis of fullerides, namely compounds in which solid C₆₀ is doped with alkali or alkaline-earth metals. These materials are obtained through various methods, such as solid-state reactions, chemical vapor deposition (CVD), and solution-phase synthesis. Fullerides exhibit fascinating physical behaviors, including superconductivity with relatively high critical temperatures (up to 38 K in Cs₃C₆₀) and a notable capacity to adsorb and absorb hydrogen, making them attractive candidates for future energy storage and conversion technologies.
Graphene
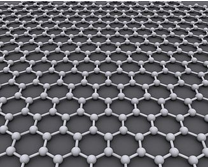
Graphene is a single atomic layer of carbon atoms arranged in a hexagonal lattice, where each atom is sp²-hybridized. First isolated in 2004 by Geim and Novoselov at the University of Manchester using mechanical exfoliation from graphite, this material earned them the Nobel Prize in Physics in 2010. Thanks to its exceptional electrical conductivity, mechanical strength, and surface area, graphene is widely studied for applications in electronics, composites, sensors, gas adsorption, and hydrogen storage. At the Nanocarbon Laboratory, graphene is synthesized by oxidizing graphite to increase interlayer spacing, followed by thermal exfoliation to separate the layers through a rapid thermal shock. The resulting few-layer graphene can be used directly or functionalized with nanoparticles (e.g. metals or oxides) for use in catalysis, energy storage, and hydrogen-related applications. Alongside this method, the lab also produces Laser-Induced Graphene (LIG) by irradiating carbon-rich substrates—such as polyimide or biomass—using a focused laser beam. This solvent-free, maskless approach creates porous, conductive graphene patterns suitable for flexible electronics, supercapacitors, sensors, and water purification. The development of both exfoliated and laser-induced graphene is a core part of the laboratory work on sustainable, high-performance carbon-based materials.
Nanoporous Carbon

Biochar is a carbon-rich material produced from biomass, such as agricultural waste, wood residues, or other organic matter, through pyrolysis or pyrogasification processes. These thermochemical conversion techniques involve the decomposition of biomass at elevated temperatures in a low-oxygen or oxygen-free environment, resulting in a solid residue that is rich in carbon and typically fine-grained in texture. In the Nanocarbon Laboratory, biochar serves as a sustainable precursor for advanced carbon materials. Through chemical activation, typically involving alkaline or acidic agents followed by thermal treatment, the internal structure of the raw biochar is modified to significantly enhance its porosity and specific surface area, which can reach values as high as 3000 m²/g. This high surface area, along with the hierarchical pore structure, is essential for optimizing the electrochemical performance of the material. Thanks to these features, activated biochar is being investigated as a low-cost, renewable, and high-performance electrode material for supercapacitors, where rapid charge–discharge capability and large surface interaction with electrolytes are key. Moreover, its tunable surface chemistry makes it suitable for further functionalization, allowing integration also in solid-state hydrogen storage systems. This line of research not only supports the development of efficient energy storage devices but also contributes to circular economy strategies by transforming biomass waste into value-added nanostructured carbon materials.
