The research activity of the Energy-Storage Laboratory, in collaboration with the Nanocarbon Laboratory, is dedicated to the development of innovative technologies for energy transport, conversion, and storage. The focus lies on the use of advanced carbon nanostructures, including fullerenes, graphene-related materials, activated biochars etc. These nanomaterials, synthesized and functionalized at the Nanocarbon Lab, are integrated into prototype devices, such as next-generation ionic batteries and supercapacitors, at the Energy-Storage Lab. These prototypes are then tested under conditions that simulate real-world use, enabling the extraction of key performance metrics such as specific capacity, energy and power density, rate capability, and cycle life.
Li-ion batteries
A battery is a device that converts chemical energy into electrical energy, which can then be used to power a wide range of devices, from smartphones and laptops to electric vehicles. Today, the state-of-the-art in rechargeable battery technology is represented by lithium-ion (Li-ion) batteries, which are widely adopted due to their ability to be recharged many times and their high energy and power densities. In a Li-ion battery, lithium ions migrate from the negative electrode (anode) to the positive electrode (cathode) during discharge, and move in the reverse direction during charging (see schematic below). For safety and stability, both electrodes typically consist of lithium-intercalated materials. The cathode is usually composed of lithium transition metal oxides (e.g., containing cobalt, manganese, or nickel), while the anode is commonly made of graphitic carbon.
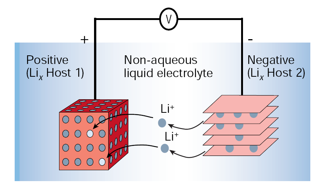
Despite their advantages, Li-ion batteries still lag behind conventional fuels such as gasoline in terms of specific energy (energy per unit mass or volume). Additionally, the limited availability of lithium resources raises concerns about scalability, particularly for large-scale applications like electric vehicles or smart grids for renewable energy storage and distribution. To overcome these limitations, research is increasingly focusing on next-generation energy storage systems that leverage advanced carbon nanostructures. Promising developments include Li-air and Li-sulfur (Li–S) batteries, which offer higher theoretical energy densities, as well as sodium-ion (Na-ion) batteries, which utilize more abundant raw materials. These emerging technologies hold significant potential to narrow the gap between batteries and fossil fuels, enabling a more sustainable energy future.
Supercapacitors
Supercapacitors are highly promising electrochemical energy storage devices that bridge the gap between conventional capacitors and rechargeable batteries. They store energy electrostatically, through charge separation at the interface between porous carbon electrodes and an electrolyte, rather than relying on faradaic (chemical) reactions. This mechanism enables rapid charge and discharge cycles, high power density, and excellent cycling stability (see figure below).
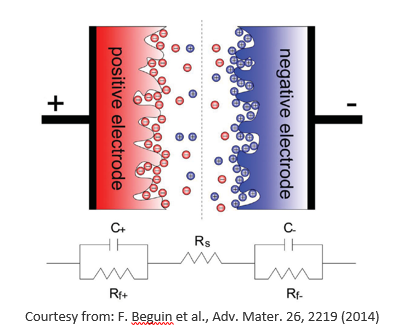
The extremely short distance between the charge layers, just a few angstroms, combined with the very high specific surface area (SSA) of porous carbon electrodes, results in capacitance values that are orders of magnitude higher than those of conventional capacitors, typically ranging from 10 to 100 F/g. Compared to rechargeable batteries, supercapacitors offer much faster charge and discharge rates (i.e., higher power density), longer cycle life, and lower production costs. However, their energy density remains significantly lower than that of Li-ion batteries, which limits their use in applications requiring long-term energy supply. To develop high-performance supercapacitors, it is essential to combine a large SSA with an optimized pore-size distribution in the electrode material, tailored to match the ionic size of the electrolyte species. This ensures efficient ion transport and maximized charge storage. Additionally, performance can be further enhanced by exploiting the so-called pseudocapacitance, a mechanism in which faradaic (redox) reactions occur at the surface of the electrode. In this case, the electrode behaves more like an ultra-thin battery, contributing additional charge storage beyond pure electrostatic accumulation.
Solid state hydrogen absorbers
Hydrogen is an exceptionally efficient energy carrier, capable of delivering three times more energy per unit mass than conventional chemical fuels. Furthermore, its combustion produces no greenhouse gas emissions, making it a key player in the transition toward clean and sustainable energy systems. However, a major barrier to the widespread adoption of a hydrogen economy lies in the challenge of developing safe, efficient, and practical hydrogen storage technologies, particularly for automotive applications. Various storage strategies have been explored, including compressed hydrogen at very high pressures and liquid hydrogen at cryogenic temperatures. While these methods are commercially available, they come with significant technical and economic limitations. The most promising performance, in terms of energy density and safety, is offered by solid-state hydrogen storage, which involves incorporating hydrogen into advanced materials such as metal hydrides, porous carbons, or complex chemical carriers. Yet, despite considerable progress, no existing solid-state material has yet met the performance targets set by industry and regulatory bodies, particularly in terms of storage capacity, reversibility, kinetics, and cost.
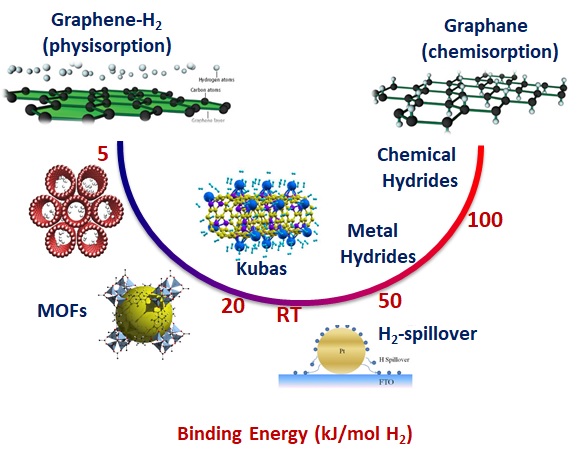
Nanoporous carbon, thanks to its hierarchical microporosity, outstanding mechanical and electronic properties, and the ability to be functionalized with nanoscale metal catalysts, offers significant potential for hydrogen storage applications. Beyond enabling substantial physisorption of hydrogen molecules, such engineered carbon materials can also facilitate enhanced interactions between hydrogen and the carbon matrix through mechanisms such as hydrogen spillover and Kubas interaction. These processes are known to tune the hydrogen binding energy to more optimal values, intermediate between the weak forces of pure physisorption and the overly strong bonds of chemisorption. As a result, they allow for reversible hydrogen uptake and release under conditions close to ambient temperature and pressure, which is essential for practical and efficient storage systems.
