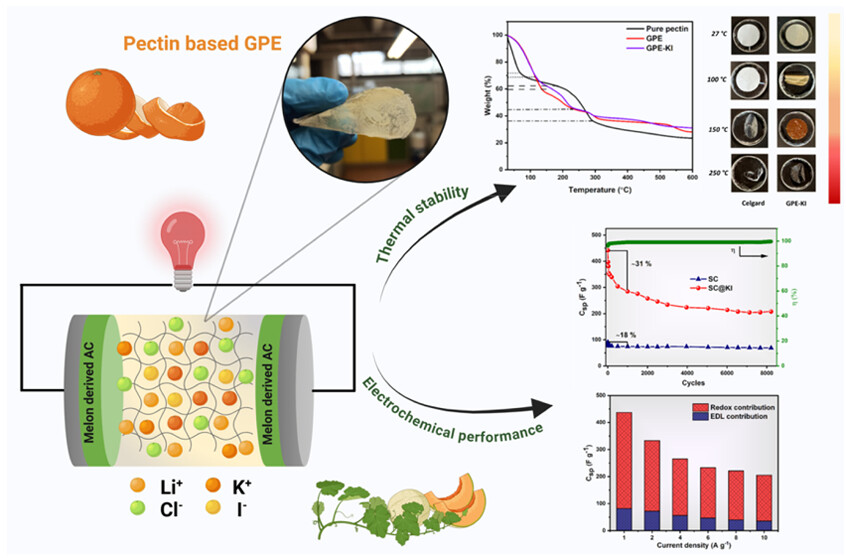
The implementation of environmentally green materials in energy storage technologies is essential to ensure a fair and ethical transition to net zero. In this work, we present a gel electrolyte (GPE) based on pectin, a biodegradable natural biopolymer synthesized by using lithium chloride (LiCl) and potassium iodide (KI) as redox additives to enhance the performance of a supercapacitor. GPE shows enhanced thermal stability and flame retardancy, as confirmed by thermogravimetric and differential scanning calorimetry analysis. The optimized redox-additive GPE exhibits high flexibility and outstanding electrochemical properties including a high ionic conductivity (σ = 43 mS cm–1) at room temperature and a wide stable potential window (∼2 V vs Ag/Ag+). The optimized GPE, with a redox additive and without, was tested with activated carbon electrodes derived from melon peel waste in symmetric supercapacitors. The addition of a redox additive to GPE films directly influences the performance of supercapacitors, leading to a 5 times increase in the specific capacitance (∼437 F g–1) and specific gravimetric energy density (∼34 Wh kg–1). The optimized supercapacitor exhibits stable cycling performance up to ∼8000 cycles by having an initial ∼31% fade in capacitance and a high Coulombic efficiency close to 99–100%.
Reproduced with permission. Copyright 2025, American Chemical Society