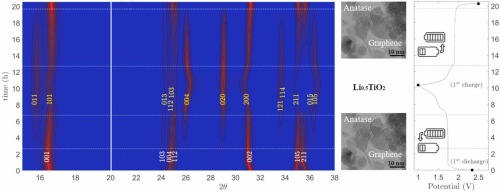
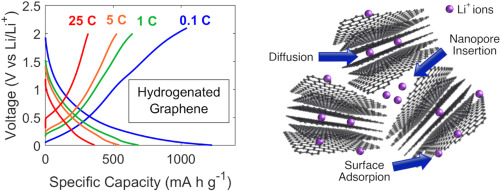
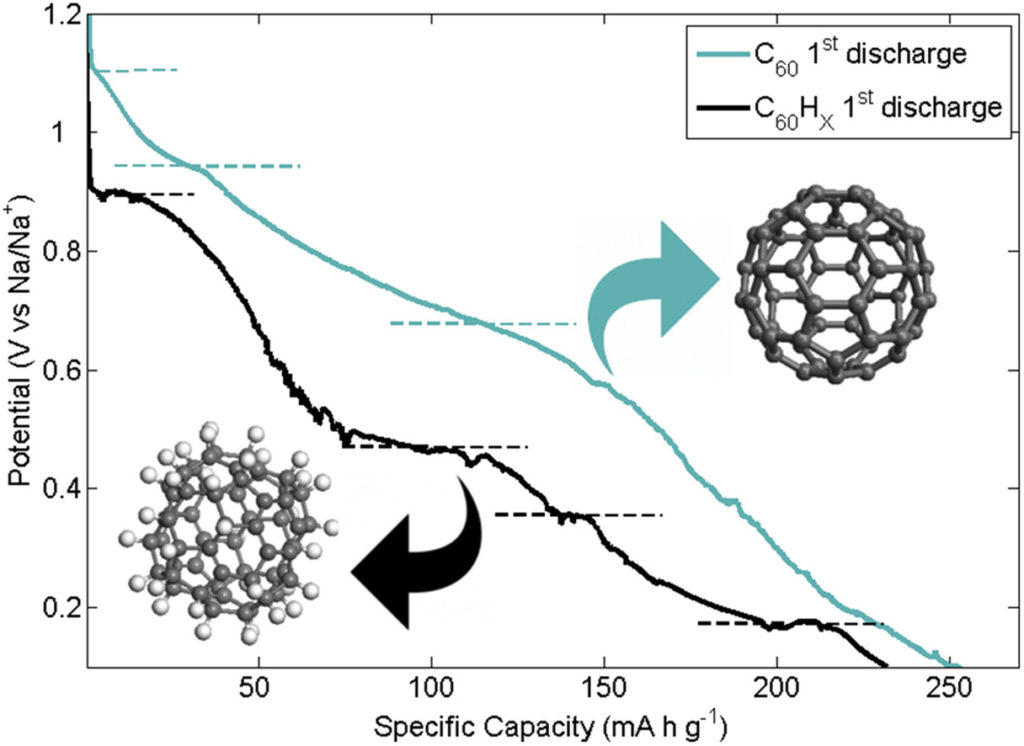
In this work, the performance of novel negative electrodes for Li-ion batteries based on defective graphene synthesized via a scalable thermal exfoliation of graphite oxide and decorated with TiO2 nanoparticles is investigated. Titania polymorphs are interesting as battery electrode materials, owing to their high cycle stability, safety, abundance and negligible solid electrolyte interphase formation and volume changes upon cycling. Defective graphene, on the other hand, can embed TiO2 nanoparticles, forming a conductive hybrid nanocomposite anode material for Li-ion batteries, with improved Li-ion and electron transport, optimising power density. Here we propose two different synthetic approaches for the decoration of graphene with TiO2 nanoparticles: I) a novel chemical route where TiO2 nanoparticles, mainly anatase, were grown on graphene in hydrothermal mild conditions and II) a physical solid-state approach where hydrothermal TiO2 nanoparticles and graphene were mixed together via high energy ball-milling. The synthesized materials were analysed via powder X-ray diffraction, micro-Raman spectroscopy and HR-TEM, while the electrodes were electrochemically tested. Operando synchrotron diffraction was conducted on half-cell to investigate phase transitions in the electrode materials. Even in presence of small amount of graphene, significant improvement in capacity, reversibility, and high-rate capability were observed. In particular, the sample obtained through the addition of 1 wt% of graphene displayed a reversible capacity of more than 180 mA h/g after prolonged and wearing cycling. This result outperforms by 327 % the reversible capacity of pure TiO2 electrode.
Reproduced with permission. Copyright 2023, Elsevier